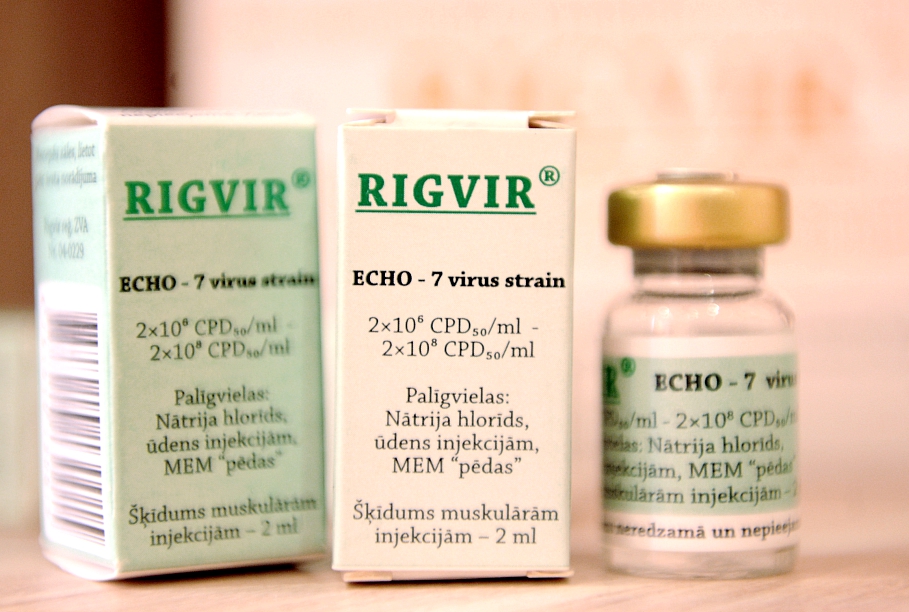
The treatment, named 'Rigvir', has long been controversial. Its developers claim it to be a major advance in the treatment of serious cancers, while skeptics maintain it is at best an unproven experiment and at worst gives expensive false hopes to terminally ill cancer patients and their families.
Currently, Rigvir is in the fifth place in the top selling list of prescription medicines in Latvia. Its sales are growing much faster than other popular medicines and last year were worth four million euros.
Crucially, it is included on the health ministry's list of reimbursable medicines, meaning public as well as private money is spent on it.
Yet as De Facto revealed, in January three professional medical organizations, including the Latvian Oncology Association, wrote to the Health Ministry expressing concern that research and publications used to establish the credentials of Rigvir treatment were of poor quality and scientifically questionable: for example in one study supposedly comparing the outcomes for two groups of patients, one group used Rigvir and the other did not, with the Rigvir patients doing considerably better than the control group. However, the Rigvir patients also used chemotherapy, while the control group did not, which should have rendered the findings highly dubious.
Oncologists asked that Rigvir be removed from the list of reimbursable medicines and the National Drug Register pending proper clinical data, and also asked for a review of the official guidelines for the treatment of melanoma involving Rigvir.
In February, the health ministry publicly pledged to review the registration of Rigvir and its inclusion in the list of reimbursable medicines. However, De Facto uncovered that a decision to ignore the oncologists' concerns had already been made in the spring.
"One of the key issues to change anything in drug registration or compensation conditions is clear evidence that the medicine is or is not effective and safe; that there are or are not side effects," Minister of Health Anda Čaksa told De Facto.
"A letter by some social group saying they don't like something - it's just not helpful. Show us data that this medicine does not work for your patients. We don't have anything to react to at the moment," said Health Minister Anda Čakša.
Karlis Urbans, Development Director of Rigvir Holding which aims to commercialize the treatment, declined to comment in detail on the oncologists' concerns but did signal that rigorous clinical research was planned for the future.
Yet in a remarkable phrase he insisted the company's own anecdotal evidence was enough to establish that Rigvir works:
"We do not need scientific evidence to see its effectiveness."
As well as manufacturing Rigvir for distribution via medical professionals, the team behind Rigvir operates the International Virotherapy Center in Riga, which trumpets the potential for this form of treatment direct to potential patients.
It has been approved for use in Armenia and Georgia in addition to Latvia. Its main claims to clinical legitimacy are based on studies summarised HERE. Meanwhile the Virotherapy Center's website shows teams of doctors from Uzbekistan, Australia, Mexico, Sri Lanka and elsewhere arriving "to complete virotherapy training course and receive the certificate of compliance."
About 300 people are compensated for using Rigvir each year in Latvia, generating sales of €4m.
Rigvir was registered in the state register of medicines a few days prior to Latvia's entry in the EU in 2004 but has not yet been approved for use elsewhere in the European Union. Approval seemed to move closer last year, but in an unusual move, regulators backtracked on an initial claim that Rigvir could have dramatic impacts on cancer treatment.
#CORDIS in coordination with @EU_EASME + @H2020SME decided to remove this news article; project under investigation https://t.co/3Tx7GMVqt9
— EU Research Results (@CORDIS_EU) September 8, 2016
The latest state of play with regard to Rigvir and 50,000 euros of financial assistance it has received from the European Commission can be read HERE.
While officials and the developers of Rigvir regard it as a major step forward in the battle against cancer, it is not only oncologists who have their doubts. A long English-language piece on the skepticisms.lv website by Eduards Ritums traces the history of Rigvir and examines it from a skeptical angle.
Latvia's official tourism portal even touts the country as a destination for Virotherapy tourism, saying that Latvia's oncologists are among the best in Europe -- the same oncologists who expressed doubts in their letter about using Rigvir.
"Latvia offers a unique method of treatment for oncological diseases, utilising viral therapy based on RIGVIR.... It has passed all phases of clinical trials, and is registered in Latvia (Reg. No. 04-0229), and is also available in Latvian pharmacies," says the Latvian Tourism Development Agency.
Nor does the praise from official circles end there. In May, around about the time the Health Ministry chose to ignore the ocologists' concerns, the national patent office awarded a trophy to LATIMA LLC, the holding company with the commercial rights to Rigvir.
In a final aside, world boxing champion Mairis Briedis is "head of security of Rigvir Holding" according to the International Virotherapy Center.
You can see the full De Facto report (in Latvian) via the link below.
.@veselibasmin neieklausās onkologos un iestājas par “Rigvir” atrašanos kompensējamo zāļu sarakstā. Plašāks stāsts: https://t.co/EbgR46EX9G pic.twitter.com/Sb8zAnsstu
— De Facto (@ltvdefacto) September 4, 2017